Astrazeneca vaccine ‘safe and effective’, study finds
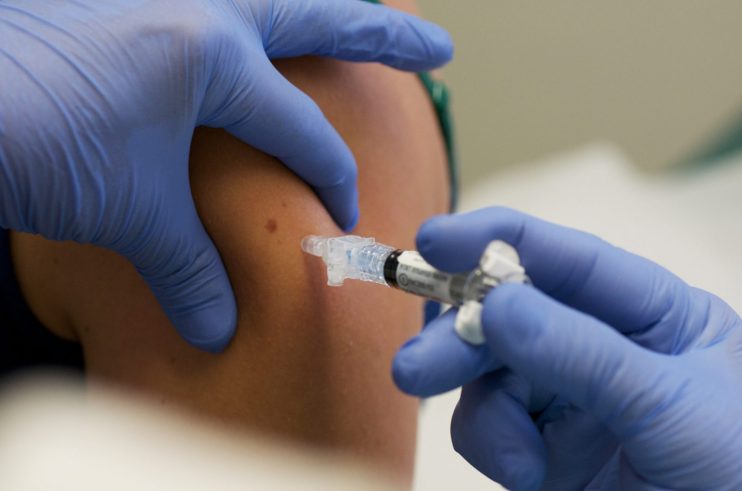
The Astrazeneca/Oxford University vaccine is safe and effective in vaccinating people against coronavirus, independent analysis has confirmed.
In a new study published in the Lancet medical journal, researchers said the jab will have a “big impact” on the coronavirus pandemic, as the UK lines up for widespread rollout of the vaccine.
The paper is the first peer-reviewed analysis of phase three data from the Astrazeneca vaccine trial of more than 20,000 people.
Professor Andrew Pollard, director of the Oxford Vaccine Group and chief investigator of the Oxford Vaccine Trial, said: “Today, we have published the interim analysis of the phase three trial and show that this new vaccine has a good safety record and efficacy against the coronavirus.
“We are hugely grateful to our trial volunteers for working with us over the past eight months to bring us to this milestone.”
The Astrazeneca vaccine presents significant logistical advantages over other alternatives including the Pfizer/Biontech jab. It does not need to be kept at ultra low temperatures and can be manufactured in large quantities at minimal cost.
The UK has hedged its bets on the efficacy of the Astrazeneca vaccine, ordering 100m doses of the drug, compared to 40m of the Pfizer/Biontech vaccine and just 7m of the Moderna vaccine.
Professor Sarah Gilbert, Professor of Vaccinology at the University of Oxford, described today as “probably the best day of 2020”.
She said “Following the demonstration of vaccine efficacy in many preclinical studies, we now have clear evidence of efficacy in the trial results presented in a peer-reviewed publication today.”
Astrazeneca drew disappointment last month after announcing that its Covid vaccine proved 70 per cent effective in trials, compared to the 95 per cent success rates of both the Pfizer and Moderna jabs.
However, effectiveness rose to 90 per cent in a group of volunteers who were given an initial half dose followed by a full dose one month later.
The difference lies in the genetic make-up of the different vaccine types. Both Pfizer and Moderna’s are based on novel messenger RNA technology, while Astrazeneca’s is made from a weakened version of a common cold virus that’s genetically changed to make it unable to grow in humans.
Astrazeneca has pledged it will not make a profit on the vaccine during the pandemic, and has reached agreements with governments and international health organisations to sell the drug at a price tag of between $4 and $5, depending on local charges.
That paves the way for a feasible exit from the coronavirus crisis for developing countries, at a time when the world’s wealthiest countries have gobbled up pre-orders of vaccine supplies.
Pfizer’s vaccine, meanwhile, costs about $20 a dose, and Moderna’s comes with a price tag of around $15 to $25.
It comes as health secretary Matt Hancock today welcomed Britain’s “V-day”, as the NHS started mass rollout of the Pfizer vaccine.
Ninety-year-old Margaret Keenan this morning became the first person in the world to receive a coronavirus vaccine outside of a trial, marking the start of Britain’s largest vaccination programme in history.
“If we manage to do that for everybody who is vulnerable to this disease, then we can move on and we can return to normal,” said Hancock.